GAVRETO Dosing
GAVRETO offers patients a once-daily dosing schedule
Start your patients at the recommended 400-mg dose1
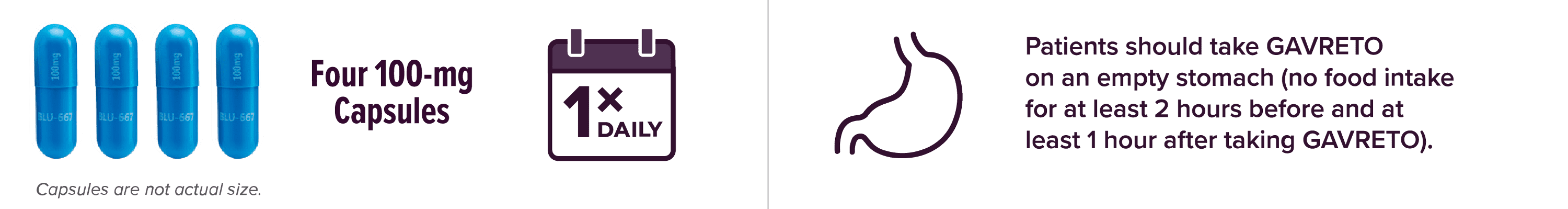
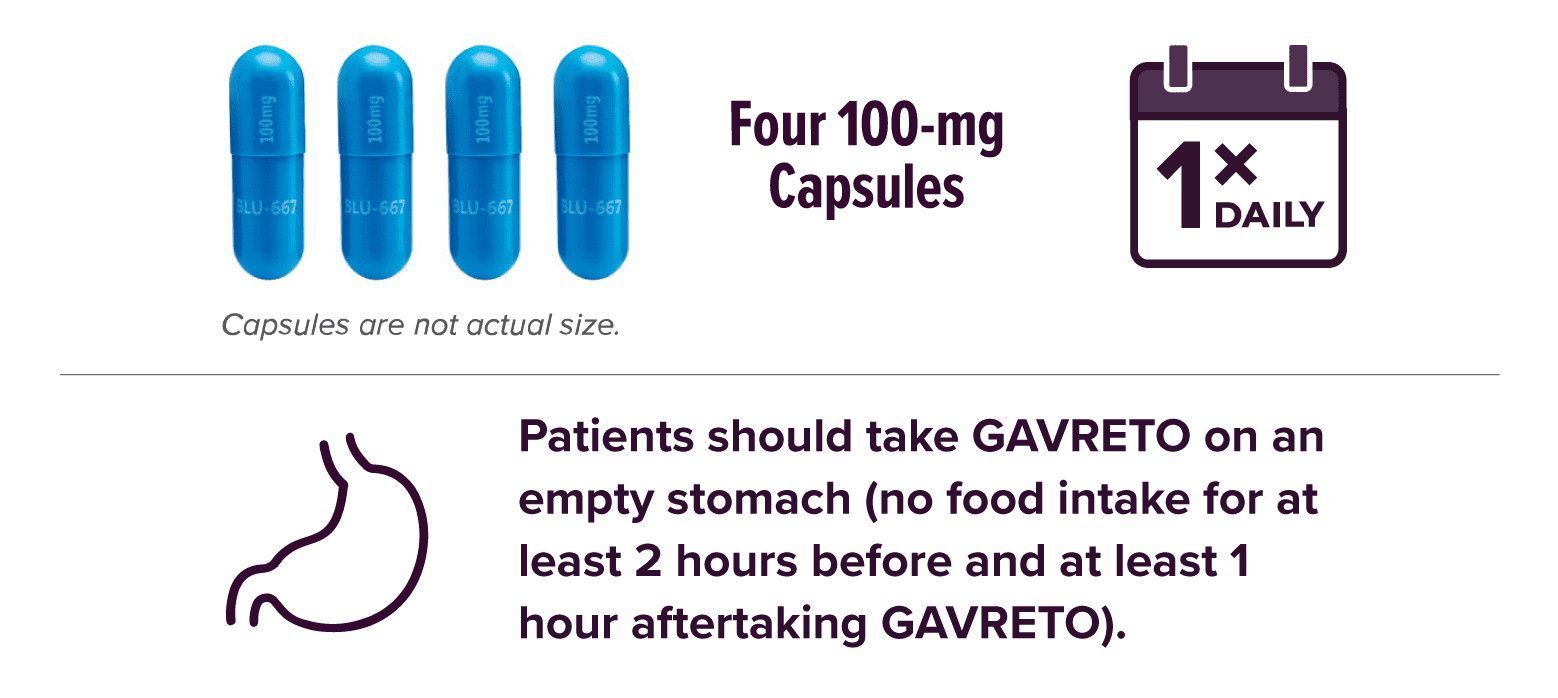
- Continue treatment until disease progression or until unacceptable toxicity
- If a dose of GAVRETO is missed, it can be taken as soon as possible on the same day. Resume the regular daily dose schedule for GAVRETO the next day
- Advise patients not to take an additional dose if vomiting occurs after taking GAVRETO but to continue with the next dose as scheduled
- Select patients for treatment with GAVRETO based on the presence of a RET gene fusion (NSCLC or thyroid cancer)
NSCLC=non–small cell lung cancer; RET=rearranged during transfection.
Recommended dosage reductions for adverse reactions1
GAVRETO's single dosage strength allows for convenient dose reductions.

Capsules are not actual size.
Permanently discontinue GAVRETO in patients who are unable to tolerate 100 mg taken orally once daily.
In exposure-response analyses, clinically improved outcomes were associated with starting at the recommended 400 mg dose of GAVRETO compared to starting at lower doses (≤300 mg QD)1,2
Exposure-response analyses were conducted on data obtained from patients in the phase 1/2 ARROW study, including patients with RET fusion-positive NSCLC. This consisted of dose escalation (phase 1, doses from 30-600 mg QD or BID) and dose expansion (phase 2, dose of 400 mg QD) phases. The exposure-response analyses were designed to evaluate the relationships between exposure and efficacy/safety outcomes, and to assess and adjust for covariate effects.2
Recommended dosage modifications for GAVRETO for adverse reactions1
| Adverse Reaction | Severity* | Dosage Modification |
| ILD/Pneumonitis | Grade 1 or 2 | Withhold GAVRETO until resolution. Resume by reducing the dose (see dose reduction table). Permanently discontinue GAVRETO for recurrent ILD/pneumonitis. |
| Grade 3 or 4 | Permanently discontinue for confirmed ILD/pneumonitis. | |
| Hypertension | Grade 3 | Withhold GAVRETO for Grade 3 hypertension that persists despite optimal antihypertensive therapy. Resume at a reduced dose when hypertension is controlled. |
| Grade 4 | Discontinue GAVRETO. | |
| Hepatotoxicity | Grade 3 or Grade 4 | Withhold GAVRETO and monitor AST/ALT once weekly until resolution to Grade 1 or baseline. Resume at reduced dose. For recurrent events at Grade 3 or higher, discontinue GAVRETO. |
| Hemorrhagic Events | Grade 3 or Grade 4 | Withhold GAVRETO until recovery to baseline or Grade 0 or 1. Discontinue GAVRETO for severe or life-threatening hemorrhagic events. |
| Other Adverse Reactions | Grade 3 or 4 | Withhold GAVRETO until improvement to ≤Grade 2. Resume at reduced dose. Permanently discontinue for recurrent Grade 4 adverse reactions. |
*Adverse reactions graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03.
ILD=interstitial lung disease.
Adverse event monitoring and coadministration
Monitor patients for the following adverse events1:
- ILD/pneumonitis: Monitor patients for pulmonary symptoms indicative of ILD/pneumonitis (such as dyspnea, cough, and fever)
- Hypertension: Do not initiate GAVRETO in patients with uncontrolled hypertension. Optimize blood pressure prior to initiating GAVRETO. Monitor blood pressure after 1 week, at least monthly thereafter and as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate
- Hepatotoxicity: Monitor AST and ALT prior to initiating GAVRETO, every 2 weeks during the first 3 months, then monthly thereafter and as clinically indicated
Avoid coadministration of GAVRETO with any of the following1,3:
- Strong CYP3A inhibitors, such as ritonavir
- Moderate CYP3A inhibitors, such as amlodipine
- P-gp inhibitors, such as clarithromycin
- Combined P-gp and strong CYP3A inhibitors, such as verapamil
- Combined P-gp and moderate CYP3A inhibitors, such as amiodarone
If coadministration with any of the above inhibitors cannot be avoided, reduce the current dose of GAVRETO as recommended in the Prescribing Information. After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume GAVRETO at the dose taken prior to initiating the inhibitor.1
Avoid coadministration of GAVRETO with any of the following1,3:
- Strong CYP3A inducers, such as rifampin
- Moderate CYP3A inducers, such as St. John's wort
If coadministration with any of the above inducers cannot be avoided, increase the current dose of GAVRETO as recommended in the Prescribing Information, starting on Day 7 of coadministration of GAVRETO with the inducer. After the inducer has been discontinued for at least 14 days, resume GAVRETO at the dose taken prior to initiating the inducer.1
CYP3A=cytochrome P450 3A; P-gp=P-glycoprotein.
Download the Dosing & Administration Guide
Take the GAVRETO Dosing and Administration Guide with you for an easy referral for your practice.
GAV_LNG-25017 0525
References:
1. GAVRETO® [Package insert], South San Francisco, CA: Rigel Pharmaceuticals, Inc.
2. GAVRETO: Data on file, Rigel Pharmaceuticals, Inc. June 2024.
3. Kassir N, McDougall D, Kuruvilla D, et al. Exposure-response relationships for pralsetinib in patients with RET-altered thyroid cancer or RET fusion-positive nonsmall cell lung cancer. J Clin Pharmacol. 2024;64(6):685-696. doi:10.1002/jcph.2409
4. Wu S, Hoang HB, Yang JZ, et al. Drug-drug interactions in pulmonary arterial hypertension. Chest. 2022;162(6):1360-1372.