Clinical Trial Information for GAVRETO
Study design in the RET+ NSCLC population1,29
Efficacy and safety with GAVRETO (400 mg orally once daily) were evaluated in patients with RET fusion+ mNSCLC in the ARROW study, a phase 1/2, nonrandomized, open-label, single-arm, multicohort, multicenter clinical trial. Patients with asymptomatic central nervous system metastases, including patients with stable or decreasing steroid use within 2 weeks prior to study entry, were enrolled.
The major efficacy outcome measures were overall response rate (ORR) and duration of response (DoR), as assessed by a blinded independent central review (BICR) according to RECIST v1.1.
mNSCLC=metastatic non–small cell lung cancer; RECIST=Response Evaluation Criteria in Solid Tumors; RET+=rearranged during transfection positive.
GAVRETO was studied in first-line and previously treated RET+ mNSCLC patient populations similar to those seen in real-world practice1,30
| Treatment-naïve patients (n=107) | Previously platinum-treated patients (n=130) | |
|---|---|---|
| Median age | 62 years (30-87) | 59 years (26-85) |
| Gender | 53% female, 47% male | 51% female, 49% male |
| Race/ethnicity | 49% White, 45% Asian, 3% Hispanic/Latino | 40% White, 50% Asian, 5% Hispanic/Latino |
| ECOG status | 0-1: 99% 2: - | 0-1: 95% 2: 4% |
| RET fusion partner | 71% KIF5B, 18% CCDC6 | 70% KIF5B, 19% CCDC6 |
| History of or current CNS metastases at baseline | 28% | 41% |
| Prior therapy type* | - | 42% PD-1/PD-L1 inhibitor 27% kinase inhibitors |
| Patient identification | 68% NGS
| 80% NGS
2% other |
*Previously platinum-treated patients received a median of 2 prior systemic therapies (range 1-6).
CNS=central nervous system; ECOG=Eastern Cooperative Oncology Group; FISH=fluorescence in situ hybridization; NGS=next-generation sequencing; PD-1=programmed cell-death protein 1; PD-L1=programmed death-ligand 1.
Clinical Results for GAVRETO
Efficacy results in treatment-naïve patients1,31
Overall Response Rate (n=107)

78% ORR
(95% CI: 68%-85%)
Duration of and Time to Response (n=83)1,31

- 45% of patients continued to respond to treatment at ≥12 months*
- Median time to first response was 1.8 months
(range: 0.9 months-6.1 months)31
Efficacy results in previously platinum-treated patients1,31
Overall Response Rate (n=130)

63% ORR
(95% CI: 54%-71%)
Duration of and Time to Response (n=82)1,31

- 66% of patients continued to respond to treatment at ≥12 months*
- Median time to first response was 1.8 months
(range: 1.3 months-11.4 months)31
Patients enrolled by July 11, 2019. Data cutoff: March 4, 2022.
*Based on observed DoR.
CI=confidence interval; CR=complete response; DoR=duration of response; NE=not estimable; ORR=overall response rate; PR=partial response.
Consistent responses were observed with GAVRETO, including CNS activity, across previously treated subgroups1
CNS Activity
Brain metastases at baseline (n=10)†

DoR at ≥6 months: 71%
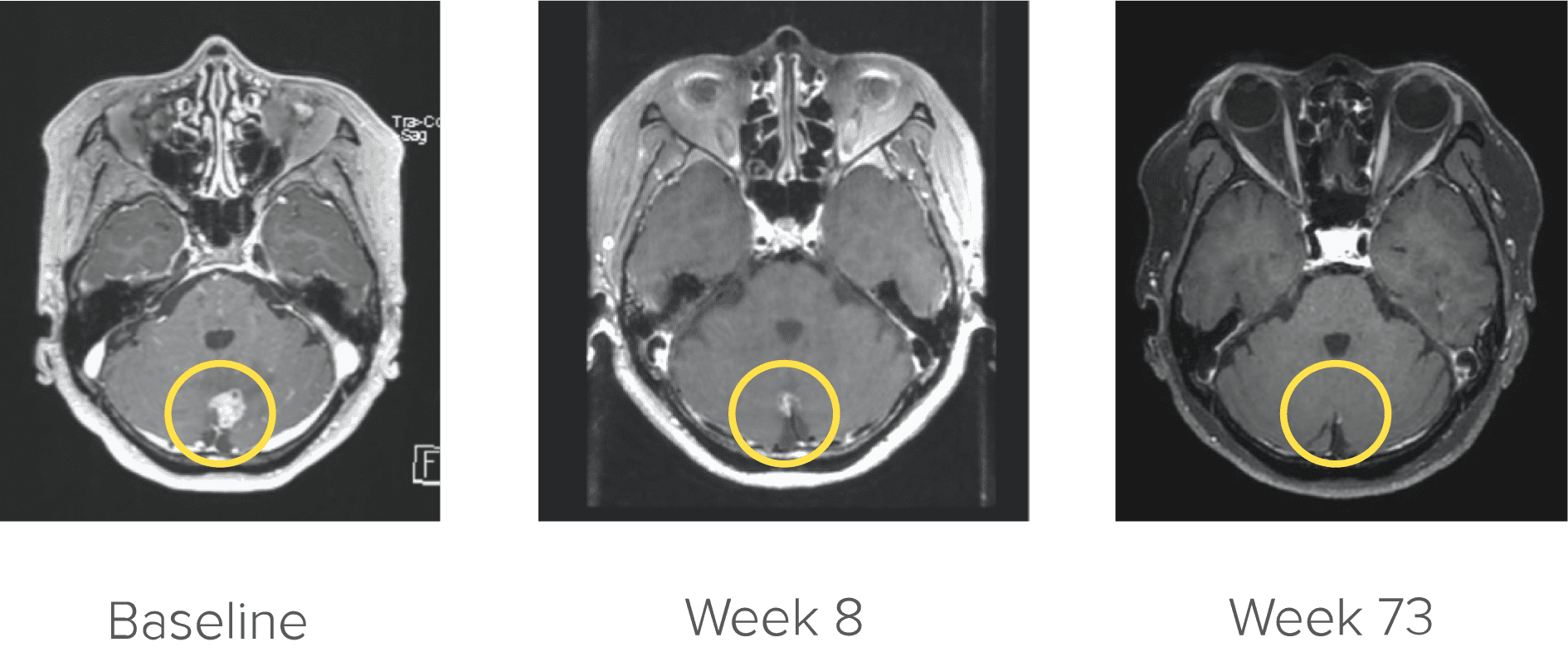
Courtesy of Dr. Kim. Image is from a patient in the ARROW trial who achieved complete CNS response. Image is for illustrative purposes only; individual results may vary. According to the protocol, images were to be performed every 8 weeks (±7 days).31
†No patients received radiation therapy (RT) to the brain within 2 months prior to study entry.
Prior PD-1/PD-L1 Inhibitor
Exploratory Analysis

Disease control rate in treatment-naïve and previously treated mNSCLC patients31
Disease control rate (DCR), a prespecified secondary endpoint, was assessed in the subsets of patients in the efficacy populations with sufficient evidence of a RET fusion and baseline measurable disease confirmed on blinded independent central review. DCR is defined as ORR (CR + PR) + SD.32
- Stable disease (SD) is defined as neither sufficient shrinkage to qualify for PR or CR nor sufficient increase to qualify for progressive disease (PD), per RECIST v1.132,33
- Importantly, SD can reflect the natural history of the disease and may not be due to a direct therapeutic effect. Therefore, please interpret these results with caution
Treatment-naïve patients (n=107)

Previously platinum-treated patients (n=130)

Patients enrolled by July 11, 2019. Data cutoff: March 4, 2022.
See RET+ mNSCLC safety for GAVRETO
Explore the safety results for all RET+ mNSCLC patients studied.
GAV_LNG-24009 0924
References:
1. GAVRETO® [Package insert], South San Francisco, CA: Rigel Pharmaceuticals, Inc.
29. Phase 1/2 study of the highly-selective RET inhibitor, pralsetinib (BLU-667), in participants with thyroid cancer, non-small cell lung cancer, and other advanced solid tumors (ARROW). https://clinicaltrials.gov/ct2/show/NCT03037385. Accessed May 7, 2024.
30. Hess LM, Han Y, Zhu YE, Bhandari NR, Sireci A. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer. 2021;21(28):1-12.
31. GAVRETO: Data on file, Rigel Pharmaceuticals, Inc. June 2024.
32. Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959-969.
33. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1–update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132-137.