Efficacy data for GAVRETO
GAVRETO was studied in patient populations representative of those seen in the real world1,2
Efficacy and safety with GAVRETO (400 mg orally once daily) were evaluated in patients with RET fusion-positive mNSCLC in the ARROW study, a phase 1/2, nonrandomized, open-label, single-arm, multicohort, multicenter clinical trial. Patients with asymptomatic central nervous system metastases, including patients with stable or decreasing steroid use within 2 weeks prior to study entry, were enrolled.1,3
mNSCLC=metastatic non–small cell lung cancer; RET=rearranged during transfection.
Demographic characteristics at baseline1
| Treatment-naïve patients (n=107) | Previously platinum-treated patients (n=130) | |
|---|---|---|
| Median age | 62 years (30-87) | 59 years (26-85) |
| Gender | 53% female 47% male | 51% female 49% male |
| Race/ethnicity | 49% White 45% Asian 3% Hispanic/Latino | 40% White 50% Asian 5% Hispanic/Latino |
| ECOG status | ||
| 0-1 | 99% | 95% |
| 2 | - | 4% |
| History of or current CNS metastases at baseline | 28% | 41% |
| RET fusion partner | ||
| KIF5B | 71% | 70% |
| CCDC6 | 18% | 19% |
| Prior therapy type* | ||
| PD-1/PD-L1 inhibitor | - | 42% |
| Kinase inhibitors | - | 27% |
| Patient identification | 68% NGS (30% tumor sample, 17% blood or plasma, 22% unknown) 19% FISH | 80% NGS (37% tumor sample, 15% blood or plasma, 28% unknown) 13% FISH 2% other |
*Previously platinum-treated patients received a median of 2 prior systemic therapies (range 1-6).1
CNS=central nervous system; ECOG=Eastern Cooperative Oncology Group; FISH=fluorescence in situ hybridization; NGS=next-generation sequencing; PD-1=programmed cell-death protein 1; PD-L1=programmed death-ligand 1.
GAVRETO is an approved therapy for both treatment-naïve and previously treated mNSCLC patients.
GAVRETO demonstrated strong and durable response regardless of prior treatment
Efficacy results in treatment-naïve patients1,4
Overall Response Rate (n=107)1

78% ORR*
(95% CI: 68%-85%)
Duration of and Time to Response* (n=83)1,4

- 45% of patients continued to respond to treatment at ≥12 months1†
- Median time to first response was 1.8 months
(range: 0.9 months-6.1 months)4
Disease control rate in treatment-naïve patients3
Treatment-naïve patients (n=107)‡

‡Disease control rate (DCR), a secondary endpoint, was assessed in the subsets of patients in the efficacy populations with sufficient evidence of a RET fusion and baseline measurable disease confirmed on blinded independent central review. DCR is defined as ORR (CR + PR) + SD.5
- Importantly, SD can reflect the natural history of the disease and may not be due to a direct therapeutic effect. Therefore, please interpret these results with caution
SD=stable disease.
Efficacy results in previously platinum-treated patients1,4
Overall Response Rate (n=130)1

63% ORR*
(95% CI: 54%-71%)
Duration of and Time to Response* (n=82)1,4

- 66% of patients continued to respond to treatment at ≥12 months1†
- Median time to first response was 1.8 months
(range: 1.3 months-11.4 months)4
Patients enrolled by July 11, 2019. Data cutoff: March 4, 2022.
*ORR and DoR were assessed by BICR, according to RECIST v1.1.
†Based on observed DoR.
BICR=blinded independent central review; CI=confidence interval; CR=complete response; DoR=duration of response; NE=not estimable; ORR=overall response rate; PR=partial response; RECIST=Response Evaluation Criteria in Solid Tumors.
Patients with brain metastases and prior PD-L1 treatment benefited from GAVRETO1
CNS ACTIVITY1
Brain metastases at baseline (n=10)‡
70%
of previously platinum-treated patients with measurable disease had a response
71%
DoR
≥6 months
‡No patients received radiation therapy (RT) to the brain within 2 months prior to study entry.
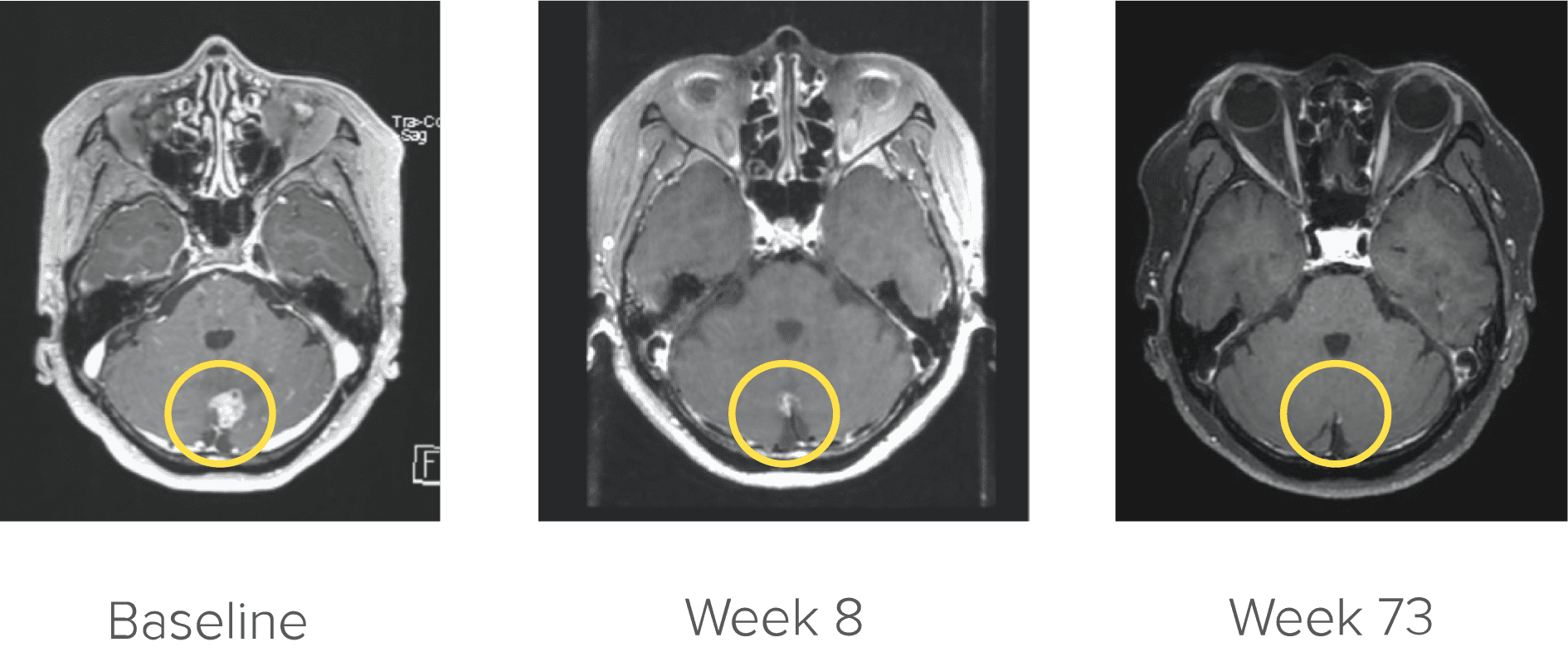
Courtesy of Dr. Kim. Image is from a patient in the ARROW trial who achieved complete CNS response. Image is for illustrative purposes only; individual results may vary. According to the protocol, images were to be performed every 8 weeks (±7 days).4
PRIOR PD-1/PD-L1 INHIBITOR
EXPLORATORY ANALYSIS1
(n=54)
59% ORR
(95% CI: 45%-72%)
Median DoR
22.3% months
(95% CI: 8.0 months-NE)
GAVRETO demonstrated strong and durable response regardless of prior treatment
See RET+ mNSCLC safety for GAVRETO
Explore the safety results for all RET+ mNSCLC patients studied.
GAV_LNG-25038 1125
References:
1. GAVRETO® [Package insert], South San Francisco, CA: Rigel Pharmaceuticals, Inc.
2. Hess LM, Han Y, Zhu YE, Bhandari NR, Sireci A. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer. 2021;21(28):1-12.
3. Phase 1/2 study of the highly-selective RET inhibitor, pralsetinib (BLU-667), in participants with thyroid cancer, non-small cell lung cancer, and other advanced solid tumors (ARROW). https://clinicaltrials.gov/ct2/show/NCT03037385. Accessed May 7, 2025.
4. GAVRETO: Data on file, Rigel Pharmaceuticals, Inc. June 2024.