About GAVRETO
Mechanism of Action (MOA)
In preclinical studies, pralsetinib was designed for potent and selective inhibition of RET1,28
In cellular assays, pralsetinib inhibited RET at approximately 14-, 40-, and 12-fold lower concentrations than VEGFR2, FGFR2, and JAK2, respectively.
Pralsetinib exhibited anti-tumor activity in cultured cells and animal tumor implantation models harboring oncogenic RET fusions or mutations, including KIF5B-RET, CCDC6-RET, RET M918T, RET C634W, RET V804E, RET V804L, and RET V804M.
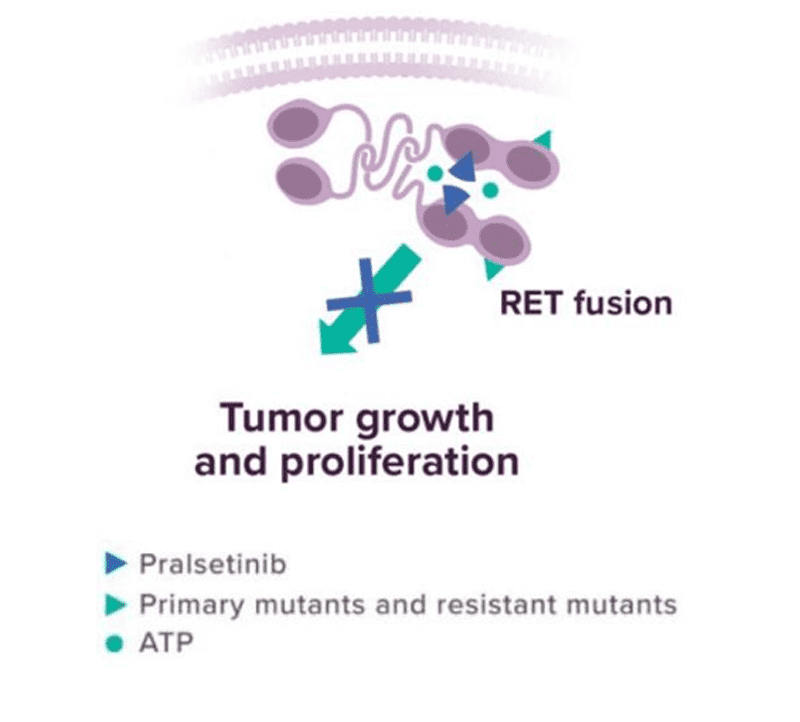
Pralsetinib prolonged survival in mice implanted intracranially with tumor models expressing KIF5B-RET or CCDC6-RET.
ATP=adenosine triphosphate; FGFR2=fibroblast growth factor receptor 2; JAK2=Janus kinase 2; RET=rearranged during transfection; VEGFR2=vascular endothelial growth factor receptor 2.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)
Pralsetinib is a NCCN Guidelines®–recommended treatment option3
NCCN Guidelines recommend pralsetinib (GAVRETO) as a NCCN Category 2A* systemic treatment option for structurally persistent/recurrent locoregional or distant metastatic RET fusion-positive PTC not amenable to RAI therapy3
*See the NCCN Guidelines for thyroid carcinoma for detailed recommendations, including other preferred options.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
PTC=papillary thyroid cancer; RAI=radioactive iodine; RECIST=Response Evaluation Criteria in Solid Tumors.
See RET+ advanced thyroid cancer data
Uncover the results for patients treated with a RET inhibitor.
GAV_THR-24004 0924
References:
1. GAVRETO® [Package insert], South San Francisco, CA: Rigel Pharmaceuticals, Inc.
3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thyroid Carcinoma V.3.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 2, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org.
28. Subbiah V, Gainor JF, Rahal R. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836-849.